Three component synthesis of 2-oxindole via sequential Michael addition, intramolecular cyclization and aromatization†
Abstract
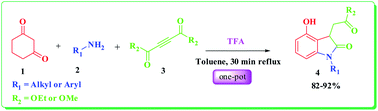
An easy metal free access to the synthesis of medicinally promising 2-oxindole derivatives via a tandem enamine formation – Michael addition – regiospecific intramolecular cyclization – aromatization sequence promoted by trifluoroacetic acid has been described. This three component reaction is regiospecific with good yield and satisfactory atom economy.

 Please wait while we load your content...
Please wait while we load your content...