Facile synthesis of 2-amino-3-bromoquinolines by palladium-catalyzed isocyanide insertion and cyclization of gem-dibromovinylanilines†
Abstract
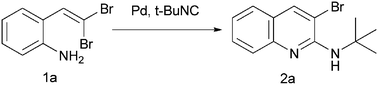
A novel and efficient synthesis of 2-amino-3-bromoquinolines through palladium-catalyzed isocyanide insertion followed by intramolecular cyclization of gem-dibromovinylanilines was developed. The reactions were carried out in 1,4-dioxane at 100 °C for 2–3 h and the corresponding products were obtained in good isolated yields.

 Please wait while we load your content...
Please wait while we load your content...