An organocatalyst from renewable materials for the synthesis of coumarins and chromenes: three-component reaction and multigram scale synthesis†
Abstract
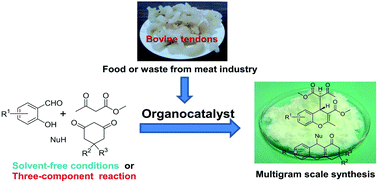
A new concept of catalysts which are prepared from renewable materials is demonstrated. It is known that amino acids (e.g., proline and hydroxyproline) are robust organocatalysts for several reactions. Bovine tendons which are proteins rich in hydroxyproline and proline were used as a source of amino acids. An acid hydrolysate of tendons (a TH catalyst) could catalyze two reactions: (i) the synthesis of coumarins and chromenes under solvent-free conditions and (ii) the synthesis of densely functionalized 4H-chromenes via a three-component reaction. Moreover, an economical and easily accessible TH catalyst is applicable in a multigram scale synthesis of coumarins and chromenes, as well as in the three-component reaction for chromene synthesis. A catalytic activity of hydroxyproline for the synthesis of 4H-chromenes via the three-component reaction was also discovered. The present work demonstrates not only the green catalysts from renewable materials, but also an environmentally benign preparation of coumarins and chromenes.

 Please wait while we load your content...
Please wait while we load your content...