Solvothermal synthesis of mesoporous manganese oxide with enhanced catalytic activity for veratryl alcohol oxidation†
Abstract
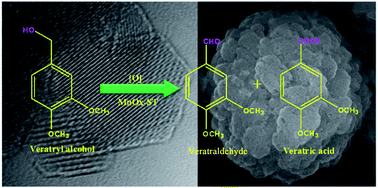
Catalyst activities of manganese and cobalt oxides prepared by solvothermal and co-precipitation methods were studied for veratryl alcohol oxidation. Manganese oxides showed higher activity performance than that of cobalt oxides irrespective of the method of preparation. The solvothermal method yielded mesoporous manganese oxide without using any template giving mixed phases of monoclinic Mn5O8 and hausmannite Mn3O4. The mesoporous manganese oxide exhibited excellent activity for liquid phase aerobic oxidation of veratryl alcohol under base free conditions, with 90% conversion and almost complete selectivity towards veratraldehyde. The detailed characterization results on morphology, size and composition of the prepared mesoporous manganese oxide obtained by XRD, XPS, H2-TPR, N2 adsorption–desorption isotherms, FE-SEM and HR-TEM techniques were used to understand the role of morphological and structural features in enhancement of the observed catalytic activity.

 Please wait while we load your content...
Please wait while we load your content...