Ring transformation of (4-chloro-5H-1,2,3-dithiazol-5-ylidene)acetonitriles to 3-haloisothiazole-5-carbonitriles†
Abstract
Ring transformation of readily prepared (4-chloro-5H-1,2,3-dithiazol-5-ylidene)acetonitriles afford 3-haloisothiazole-5-carbonitriles in good to excellent yields. The transformation can be mediated using HBr (g), HCl (g) or BnEt3NCl. Mechanisms for the transformations are discussed, together with rationalizations for the formation of side products. Furthermore, single crystal X-ray structures are provided for (Z)-2-(4-chloro-5H-1,2,3-dithiazol-5-ylidene)acetonitrile and (E)-2-bromo-2-(4-chloro-5H-1,2,3-dithiazol-5-ylidene)acetonitrile confirming the stereochemistry of the exocyclic ethene bond.
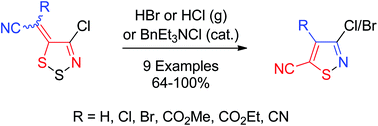

 Please wait while we load your content...
Please wait while we load your content...