A room-temperature synthesis of 2,2′-bisoxazoles through palladium-catalyzed oxidative coupling of α-isocyanoacetamides†
Abstract
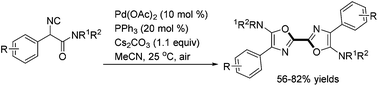
A palladium-catalyzed synthesis of symmetric and unsymmetric 2,2′-bisoxazoles starting from readily available α-isocyanoacetamides was developed. The reaction was performed at room temperature in air which acted as the sole oxidant of Pd(0). Mechanistic studies suggested that double isocyanide insertion into the Pd(II)–O bond was involved.

 Please wait while we load your content...
Please wait while we load your content...