Cationic gold(i)-catalyzed enantioselective hydroalkylation of unactivated alkenes: influence of the chloride scavenger on the stereoselectivity†
Abstract
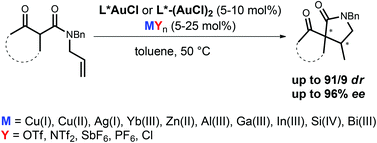
Enantioselective intramolecular additions of β-ketoamides to unactivated alkenes have been accomplished by means of gold catalysis. Chiral LAuCl complexes have been activated by various Lewis acids (Cu, Ag, Ga, In, Si, Bi etc.). The influence of these activators on the stereoselectivity has been studied.
- This article is part of the themed collection: In Celebration of Max Malacria’s 65th Birthday

 Please wait while we load your content...
Please wait while we load your content...