Abstract
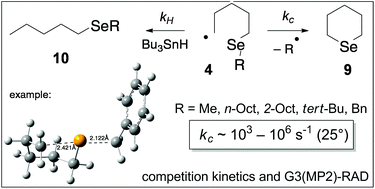
Intramolecular homolytic substitution reactions of 5-(alkylseleno)pentyl radicals 4 have been investigated by competition kinetics as well as computational techniques. B3LYP/6-31G(d) calculations predict that cyclizations of radicals 4 proceed through unremarkable transition states 11 in which the attacking and leaving radicals follow trajectories that deviate some 10–20° from co-linearity with distances in the expected 2.1–2.4 Å range. Competition kinetic experiments provide activation energies (Ea) that lie in the range: 20–36 kJ mol−1, and depend on the nature of the leaving radical, while G3(MP2)-RAD calculations provide data that are in good agreement with those obtained experimentally. Values of log(A/s−1) lie in the expected range of ∼9–11. These data provide rate constants for cyclization that span three orders of magnitude at 25°, namely: 103–106 s−1. This work also provides valuable Arrhenius data for the benzyl-substituted system 4 (R = Bn) (kc = 5.8 × 104 s−1 in benzene at 25°) and is important because the benzyl radical has become the “workhorse” for radical ring closures at selenium.
- This article is part of the themed collection: In Celebration of Max Malacria’s 65th Birthday

 Please wait while we load your content...
Please wait while we load your content...