Olefin cross-metathesis, a mild, modular approach to functionalized cellulose esters†
Abstract
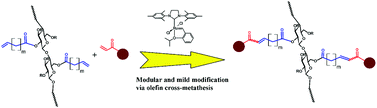
Olefin cross-metathesis has been demonstrated to be a modular pathway for synthesis of a series of functionalized cellulose esters. As a proof of concept, cellulose acetate was acylated with two terminally olefinic acid chlorides, pent-4-enoyl chloride and undec-10-enoyl chloride, providing olefin-terminated cellulose esters with different side-chain lengths. These ω-unsaturated cellulose esters were then reacted with a variety of cross-metathesis partners, including acrylic acid, methyl acrylate, 2-hydroxyethyl acrylate, poly(ethylene glycol) methyl ether acrylate, and allyl alcohols, using Hoveyda–Grubbs’ 2nd generation catalyst. Complete conversion to cross-metathesis products was achieved in reactions with acrylic acid or acrylates using 3–5 mol% catalyst at 40 °C within 1 h. We further demonstrate successful hydrogenation of these α,β-unsaturated esters and acids, thereby eliminating the potential for radical-induced crosslinking during storage.

 Please wait while we load your content...
Please wait while we load your content...