Photophysical properties and electronic structure of retinylidene–chlorin–chalcones and analogues†
Abstract
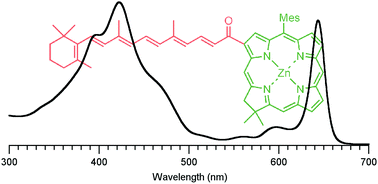
Synthetic chlorins can accommodate diverse substituents about the macrocycle perimeter. Simple auxochromes (e.g., vinyl, acetyl, phenyl) allow systematic tuning of spectral and photophysical features. More extensive spectral tailoring may be achieved by using more potent, highly conjugated substituents that themselves bring new absorption into a target spectral region, if deleterious excited-state quenching processes can be avoided. To explore such an expanded substituent space, herein the spectral and photophysical properties of four chlorin–chalcones are reported. The molecules are free base and zinc chlorins with substituents at the 13-position that include a chalcone and an extended chalcone derived by reaction of the 13-acetylchlorin with benzaldehyde and all-trans-retinal, respectively. Measurements of the spectral and photophysical properties (Φf, τs, kf, kic, kisc) are accompanied by density functional calculations that examine the characteristics of the frontier molecular orbitals. The chlorin–chalcones in nonpolar (toluene) and polar (dimethylsulfoxide) media exhibit bathochromically shifted (and intense) Qy absorption bands. The presence of the retinylidene group adds new absorption in the blue-green region where the chlorins are typically transparent; excitation in this region leads to quantitative formation of the chlorin Qy excited state. The spectral properties generally correlate with substituent effects on the frontier MOs. The four chlorin–chalcones in the solvent toluene have high fluorescence yields (0.24–0.30) and multi-nanosecond singlet excited-state lifetimes (3.7–8.4 ns), in addition to the added absorption imparted by the chalcone moiety. Collectively, the studies reported herein provide insight into the fundamental properties of chlorins and illustrate the utility of chalcones as a means of both tuning and augmenting the spectral properties of these chromophores.

 Please wait while we load your content...
Please wait while we load your content...