Synthesis and photopolymerization kinetics of 2-phenyl-benzodioxole†
Abstract
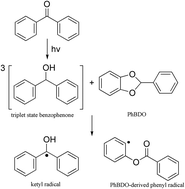
In this paper, a new photocoinitiator for free radical photopolymerization, belonging to the benzodioxole (BDO) derivatives, was synthesized and characterized, and the effect of phenyl at the 2-position of BDO was estimated. The structure of PhBDO was characterized by elemental analysis, 1H NMR and 13C NMR. Laser flash photolysis (LFP) and an electron spin resonance spin trapping technique (ESR-ST) were used to study the photochemical mechanisms. The rate of decomposition (Rd) of PhBDO in acetonitrile was studied by UV-Vis spectroscopy and the photopolymerization kinetics were monitored by real time Fourier Transform near-IR (FT-IR). The FT-IR results showed that the concentration of PhBDO and BP functionalities of the acrylates and the light intensity affected the polymerization rate and the final conversion. The results showed that the BP/PhBDO system had the same hydrogen abstraction mechanism and reactivity as the BP/BDO system. Consequently, PhBDO could also be an effective coinitiator.

 Please wait while we load your content...
Please wait while we load your content...