Synthesis of new asymmetric xanthene dyes via catalyst-free SNAr with sulfur nucleophiles†
Abstract
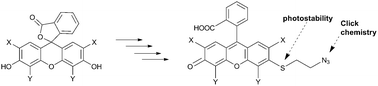
Addition of a single functional handle to the tricyclic moiety of fluorescein results in asymmetric xanthene dyes. Our synthesis of a new class of asymmetric xanthenes proceeds via an unusual SNAr with sulfur nucleophiles on electron rich aromatic xanthenes scaffolds in the absence of a metal catalyst. The resulting 3′-thioethers exhibit high photostability and are conveniently converted into reactive dyes for macromolecule labelling.

 Please wait while we load your content...
Please wait while we load your content...