Remote conformational control of a molecular switch via methylation and deprotonation†
Abstract
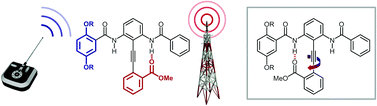
Exacting control over conformation in response to an external stimulus is the central focus of molecular switching. Here we describe the synthesis of a series of diphenylacetylene-based molecular switches, and examine their response to covalent modification and deprotonation at remote phenolic positions. A complex interplay between multiple intramolecular hydrogen bond donors and acceptors determines the global conformation.

 Please wait while we load your content...
Please wait while we load your content...