Approaches to design non-covalent inhibitors for human granzyme B (hGrB)†
Abstract
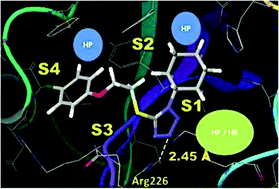
A structure-based design campaign for non-covalent small molecule inhibitors of human granzyme B was carried out by means of a virtual screening strategy employing three constraints and probe site-mapping with FTMAP to identify ligand “hot spots”. In addition, new scaffolds of diverse structures were subsequently explored with ROCS shape-based superposition methods, following by Glide SP docking, induced fit docking and analysis of QikProp molecular properties. Novel classes of moderately active small molecule blockers (≥25 μM IC50 values) from commercially available libraries were identified, and three novel scaffolds have been synthesized by multi-step procedures. Furthermore, we provide an example of a comprehensive structure-based drug discovery approach to non-covalent inhibitors that relies on the X-ray structure of a covalently bound ligand and suggest that the design path may be compromised by alternative and unknown binding poses.
- This article is part of the themed collection: Coronavirus articles - free to access collection

 Please wait while we load your content...
Please wait while we load your content...