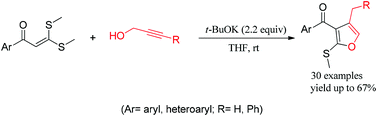
A convenient base-mediated synthesis of 3-aryol-4-methyl (or benzyl)-2-methylthio furans from α-oxo ketene dithioacetals and propargyl alcohols via domino coupling/annulations†
Abstract
A convenient base-mediated strategy to synthesize 3-aryol-4-methyl (or benzyl)-2-methylthio furans 2 (trisubstituted furans) has been developed through the domino coupling/annulations between available α-oxo ketene dithioacetals 1 and propargyl alcohols. In this strategy, these types of bases play an important role in driving the domino coupling reaction of propargyl alcohols and further intramolecular annulations to realize the target compounds. The possible mechanism for the formation of the various products is believed to involve the generation of allenes 7, followed by intramolecular annulations.

 Please wait while we load your content...
Please wait while we load your content...