Enantioselective total syntheses of the proposed structures of prevezol B and evaluation of anti-cancer activity†
Abstract
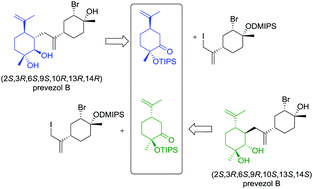
The first enantioselective total syntheses of the proposed structures of the natural product prevezol B are reported. The reported syntheses complement the previously-reported syntheses of the proposed structures of prevezol C, a stereoisomer of prevezol B. It was previously shown that the structure of the naturally occurring prevezol C had been incorrectly assigned. This work has led us to conclude that the proposed structures of prevezol B are also incorrect and major revision of both of the structures of the prevezols B and C is required. Cytotoxicity studies on the human cervical cancer cell line HeLa revealed that the synthesized prevezol B and C compounds were not active even at the highest concentration used (100 μM). However, one of the synthetic precursors was shown to have modest potency against HeLa cells (IC50 = 23.5 ± 1.8 μM).

 Please wait while we load your content...
Please wait while we load your content...