Molecular iodine-mediated reaction of 2-(2-phenylethynyl)-Morita–Baylis–Hillman adducts: an easy route to naphthyl ketones and iodo-substituted isochromenes†
Abstract
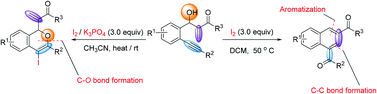
The molecular iodine-promoted reaction of 2-(2-phenylethynyl)-Morita–Baylis–Hillman adducts is reported. In the presence of I2, naphthyl ketone derivatives are produced, whereas in the presence of I2/K3PO4, iodo-substituted isochromene derivatives are produced.

 Please wait while we load your content...
Please wait while we load your content...