Facile synthesis of antimalarial 1,2-disubstituted 4-quinolones from 1,3-bisaryl-monothio-1,3-diketones†
Abstract
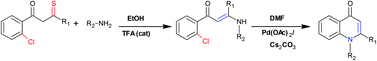
A new strategy was developed to synthesize 1,2-disubstituted 4-quinolones in good yield starting from 1,3-bisaryl-monothio-1,3-diketone substrates. The synthesized compounds were evaluated for antimalarial activity using Plasmodium falciparum strains. All compounds, except for two, showed good activity. Of these, seven compounds exhibited an excellent antimalarial activity (IC50, <2 μM). More importantly, all seven compounds were equally effective in inhibiting the growth of both chloroquine-sensitive and chloroquine-resistant strains. The cytotoxicity assessment using carcinoma and non-carcinoma human cell lines revealed that almost all synthesized compounds were minimally cytotoxic (IC50, >50 μM).

 Please wait while we load your content...
Please wait while we load your content...