Aerobic oxysulfonylation of alkenes using thiophenols: an efficient one-pot route to β-ketosulfones†
Abstract
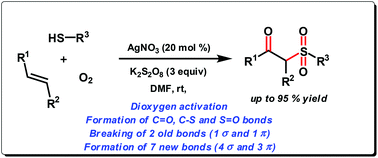
We have developed a highly efficient synthetic route to β-ketosulfones via AgNO3 catalyzed oxysulfonylation of alkenes using thiophenols in the presence of air (O2) and K2S2O8 as eco-friendly oxidants. Thiophenols have been used as sulfonylation precursors for the first time in a dioxygen activation based radical process. Moreover, the protocol also offers a new and convenient method for the synthesis of β-hydroxysulfides at room temperature without the use of any initiator.

 Please wait while we load your content...
Please wait while we load your content...