Regioselective synthesis of 3,4,5-trisubstituted 2-aminofurans†
Abstract
Three series of methyl 5-substituted 2-aminofuran-4-keto-3-carboxylates have been prepared following a multicomponent reaction strategy by the addition of an isocyanide to 4-oxo-2-butynoate in the presence of an aldehyde. The cycloaddition regioselectivity is generally high (>95%) but decreases when an electron-rich substituent is located at the butynoate 4-position.
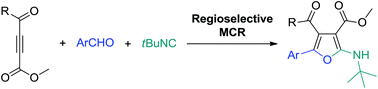

 Please wait while we load your content...
Please wait while we load your content...