Reactivity in the nucleophilic aromatic substitution reactions of pyridinium ions†
Abstract
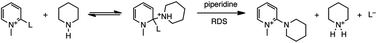
The “element effect” in nucleophilic aromatic substitution reactions (SNAr) is characterized by the leaving group order, L = F > NO2 > Cl ≈ Br > I, in activated aryl substrates. A different leaving group order is observed in the substitution reactions of ring-substituted N-methylpyridinium compounds with piperidine in methanol: 2-CN ≥ 4-CN > 2-F ∼ 2-Cl ∼ 2-Br ∼ 2-I. The reactions are second-order in [piperidine], the mechanism involving rate determining hydrogen-bond formation between piperidine and the substrate-piperidine addition intermediate followed by deprotonation of this intermediate. Computational results indicate that deprotonation of the H-bonded complex is probably barrier free, and is accompanied by simultaneous loss of the leaving group (E2) for L = Cl, Br, and I, but with subsequent, rapid loss of the leaving group (E1cB-like) for the poorer leaving groups, CN and F. The approximately 50-fold greater reactivity of the 2- and 4-cyano substrates is attributed to the influence of the electron withdrawing cyano group in the deprotonation step. The results provide another example of β-elimination reactions poised near the E2-E1cB mechanistic borderline.

 Please wait while we load your content...
Please wait while we load your content...