Iron-catalyzed tetrasubstituted alkene formation from alkynes and sodium sulfinates†
Abstract
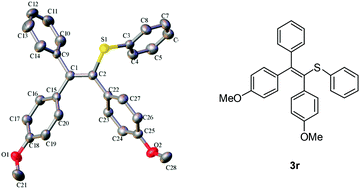
An iron-catalyzed sulfenylation and arylation of alkynes with aryl sulfinic acid sodium salts is described. Various aromatic sodium sulfinates acted both as aryl and sulfenylation reagents, affording tetrasubstituted alkenes in one pot with good yields.

 Please wait while we load your content...
Please wait while we load your content...