Design and synthesis of potent macrocyclic HIV-1 protease inhibitors involving P1–P2 ligands†
Abstract
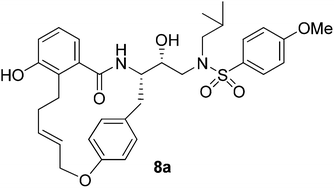
A series of potent macrocyclic HIV-1 protease inhibitors have been designed and synthesized. The compounds incorporated 16- to 19-membered macrocyclic rings between a nelfinavir-like P2 ligand and a tyrosine side chain containing a hydroxyethylamine sulfonamide isostere. All cyclic inhibitors are more potent than their corresponding acyclic counterparts. Saturated derivatives showed slight reduction of potency compared to the respective unsaturated derivatives. Compound 8a containing a 16-membered ring as the P1–P2 ligand showed the most potent enzyme inhibitory and antiviral activity.

 Please wait while we load your content...
Please wait while we load your content...