Polyalkynylanthracenes – syntheses, structures and their behaviour towards UV irradiation†
Abstract
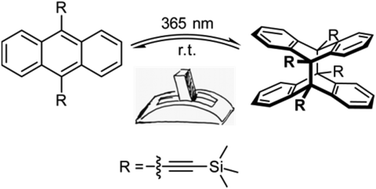
A series of bis- and tris[(trimethylsilyl)ethynyl]anthracenes (1,5-, 1,8-, 9,10- and 1,8,10-) has been synthesised by multistep (cross coupling) reactions and the behaviour of the SiMe3-functionalised alkynylanthracene derivatives towards UV irradiation was qualitatively studied by NMR spectroscopy. In the case of 9,10-bis[(trimethylsilyl)ethynyl]anthracene we observed a photodimerisation upon UV irradiation; the third example was reported for a symmetrically 9,10-difunctionalised anthracene derivative, besides those with small fluorine- and methyl-substituents. The anthracene dimerisation is completely thermally reversible and the temperature dependence of the cycloelimination reaction was studied by 1H VT-NMR experiments. The (deprotected) 1,5- and 1,8-diethynylanthracenes were converted with (dimethylamino)trimethylstannane to obtain the corresponding SnMe3-functionalised alkynes, potentially useful as highly conjugated building blocks in Stille cross coupling reactions. The new anthracene compounds were completely characterised by multinuclear NMR spectroscopy, (high resolution) mass spectrometry and – in most cases – by X-ray diffraction experiments.

 Please wait while we load your content...
Please wait while we load your content...