3,6-Substituted-1,2,4,5-tetrazines: tuning reaction rates for staged labeling applications†
Abstract
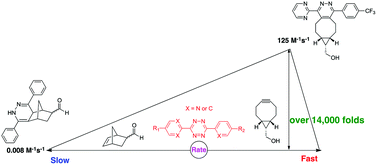
Cycloaddition reactions involving tetrazines have proven to be powerful bioorthogonal tools for various applications. Conceivably, sequential and selective labeling using tetrazine-based reactions can be achieved by tuning the reaction rate. By varying the substituents on tetrazines, cycloaddition rate variations of over 200 fold have been achieved with the same dienophile. Upon coupling with different dienophiles, such as norbornene, the reaction rate difference can be over 14 000 fold. These substituted tetrazines can be very useful for selective labeling under different conditions.

 Please wait while we load your content...
Please wait while we load your content...