Stereoselectivity through a network of non-classical CH weak interactions: a prospective study of a bicyclic organocatalytic scaffold†
Abstract
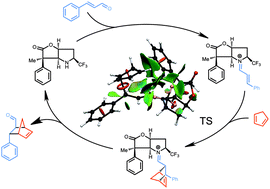
A prospective novel organocatalyst scaffold has been investigated by computational studies for its potential to induce stereoselective reactions. Density functional theory calculations (DFT) of an organocatalysed Diels–Alder reaction between cinnamaldehyde and cyclopentadiene, show that it is possible to induce selectivity by placing appropriate chemical groups on the scaffold. In the present case small substituents with strong electronic effects are found to be more effective in controlling the stereochemical induction than sterically bulky substituents. We show, using noncovalent interaction analysis (NCI plots), that the CF3 group is especially efficient in improving the stereochemical control as well as increasing the reaction rate.

 Please wait while we load your content...
Please wait while we load your content...