Perfluorinated 1H-indazoles and hydrotris(indazol-1-yl)borates. Supramolecular organization and a new synthetic procedure to form scorpionate ligands†
Abstract
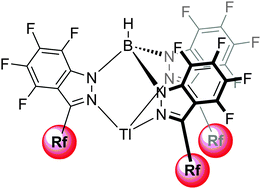
This paper describes the syntheses and full characterization of perfluorinated 1H-indazoles 2–5 and hydrotris(indazolyl)borate thallium complexes 6–9 that contain linear perfluoroalkyl chains varying from two to six carbon atoms in the 3-position. In the solid state, the perfluorinated 1H-indazoles exhibit supramolecular structures that depend on the length of the perfluoroalkyl chain. A catemer of order 3 is observed for the CF2CF3 derivative 2 (chiral space group P32), catemers of order 2 are observed for the C3F7 and C4F9 derivatives 3 (chiral space group P212121, one type of helix in the unit cell) and 4 (space group P21/n, two types of helices in the unit cell), respectively, and stacks of dimers are observed for the indazole with the longer C6F13 chain 5 (space group P21/c). The perfluorinated hydrotris(indazolyl)borate thallium complexes 6–9 [TlFn–Tp4Bo,3Rf] have been obtained by a new reaction based on the reaction of HBBr2 (generated in situ from BBr3 and Et3SiH) with the indazolates of 2–5 followed by cation exchange. The X-ray crystal structure of [TlF33–Tp4Bo,3C3F7] 7 shows that, in addition to coordination to the three nitrogens, the thallium is buried in a nest of fluorines with seven short intramolecular Tl⋯F contacts with the pendant perfluoropropyl chains. The potential of these highly fluorinated molecules to act as ligands is highlighted.

 Please wait while we load your content...
Please wait while we load your content...