Luminescence behaviour in acetonitrile and in the solid state of a series of lanthanide complexes with a single helical ligand†‡
Abstract
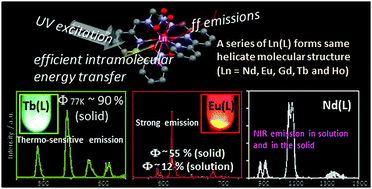
Luminescence mechanisms of EuIII, TbIII, GdIII and NdIII complexes with a hexadentate ligand (abbreviated to EuL, TbL, GdL, and NdL, respectively), which have two bipyridine moieties bridged by an ethylenediamine unit, have been examined. Our molecular design is that each complex forms a single helical polar structure based on the chelate ring to retain solubility in solutions. EuL and NdL show comparably bright emission from ff transitions both in acetonitrile solution and in the solid state. To understand the mechanism of the emission in detail, the energy level of the triplet (T) state of the ligand L has been estimated based on the phosphorescence measurements of GdL, because GdIII shows no ff emission. The donor level of the T state of L and the acceptor level of EuIII or NdIII can overlap, indicating that the excited photon localized on L has been used for the efficient ff emission, while not for ππ* emission. For TbL, the luminescence quantum yield is significantly dependent on temperature and the state: in the solid state of TbL, the quantum yield of ff emission is over 90% at 77 K, while no luminescence is observed at room temperature, and in solution TbL shows no emission. This observation suggests that the emissive f-level of TbIII and the energy donor level of the excited T state of L are in thermal equilibrium. The described lanthanide complexes are stable and retain their molecular structure even in solutions and show characteristic luminescence behaviour based on the energy relaxation process of each lanthanide ion. Furthermore the HoIII complex with L (HoL) has been prepared and its structure has been analyzed. HoL has a twisted arrangement of the bipyridine moiety surrounding HoIII due to the small ionic radius of HoIII.

 Please wait while we load your content...
Please wait while we load your content...