Dimerization hot spots in the structure of human Hsp90
Abstract
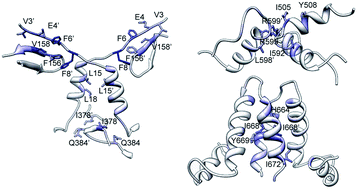
Dimerization is an essential step of the Hsp90 cycle. This work describes the results of molecular dynamics and dimerization free energy analyses performed on the structure of the human Hsp90 closed dimer. Free energy decomposition on a domain- and residue-basis highlighted different dimerization hot spots within the dimer interface that could provide binding sites for the design of allosteric inhibitors.

 Please wait while we load your content...
Please wait while we load your content...