Optimizing glucokinase activator binding kinetics to lower in vivo hypoglycemia risk†
Abstract
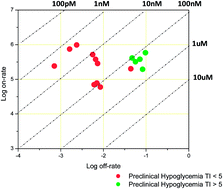
Activation of glucokinase represents a promising strategy for the treatment of type 2 diabetes; however, drug candidates have failed in clinical trials due to narrow therapeutic index between glucose-lowering efficacy and hypoglycemia. Described herein is a novel strategy for the design of next generation glucokinase activators with increased therapeutic index, which involves the optimization of activator-enzyme binding kinetics (kon, koff). This approach is based on the idea that activator binding kinetics are relevant to pharmacodynamics since the affinity of activator binding to glucokinase is cooperative with glucose such that, the rate at which an activator dissociates may influence the enzyme's sensitivity to changes in physiological glucose concentrations. This study provides a compelling example of using fast-off binding kinetics for developing safe and effective activator drugs targeting glucokinase.

 Please wait while we load your content...
Please wait while we load your content...