Investigation of novel ropinirole analogues: synthesis, pharmacological evaluation and computational analysis of dopamine D2 receptor functionalized congeners and homobivalent ligands†
Abstract
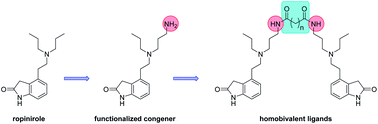
Herein, we report the development of novel functionalized congeners of ropinirole toward the design of pharmacological tools to probe structural requirements at the dopamine D2 receptor. Subsequently, we have used the functionalized amine congener 11 and synthesized and pharmacologically evaluated a series of homobivalent ligands of ropinirole with designated spacer lengths ranging from 14 to 30 atoms. The most potent homobivalent ligands (22-, 26- and 30-atom spacers) showed approximately 20- to 80-fold greater potency (EC50 = 3.9, 6.2 and 14 nM, respectively) than ropinirole (304 nM) in a [35S]GTPγS functional assay. Molecular modeling studies suggest that the observed increase in potency of the homobivalent ligands is possibly due to a bitopic binding mode involving the orthosteric site and an allosteric interaction at the dopamine D2 receptor protomer rather than bridging interactions at two orthosteric sites across a dopamine D2 receptor dimer. This research has the potential to advance the development of structurally related bitopic ligands, biomarkers such as radioligands and fluorescently labeled probes, and furnish new homo- and heterobivalent ligands towards a better understanding of the dopamine D2 receptor and potential novel treatment for Parkinson's disease.

 Please wait while we load your content...
Please wait while we load your content...