In vitro anticancer activity and SAR studies of triazolyl aminoacyl(peptidyl) penicillins†
Abstract
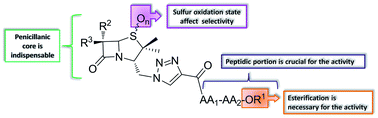
A library of triazolyl aminoacyl(peptidyl) penicillins was designed, synthesized, and evaluated for their antiproliferative activity against HeLa and B16-F0 cell lines. Structure–activity relationship studies were carried out, and minimal structural requirements were determined. Among the tested compounds, derivatives 7f, 7p and 7m demonstrated the highest anticancer activity and a promising selectivity profile against these two cell lines.

 Please wait while we load your content...
Please wait while we load your content...