Design and synthesis of a novel series of N,4-diphenylpyrimidin-2-amine derivatives as potent and selective PI3Kγ inhibitors
Abstract
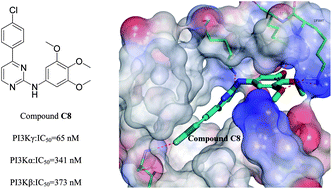
Due to the increasing evidence linking the PI3Kγ pathway to various disease states, PI3Kγ is becoming an important target for cancer treatment. Herein we designed and synthesized a novel series of N,4-diphenylpyrimidin-2-amine derivatives with low CDOCKER_INTERACTION_ENERGY and then evaluated their PI3Kγ in vitro inhibitory activities and in vitro antiproliferation assays against four human cancer cells. Among the compounds we synthesized, compound C8 (IC50 = 65 nM) demonstrated the most potent inhibitory activity against PI3Kγ kinase as well as at the cellular level, compared to the control drug TG100713 (IC50 = 127 nM). Moreover, molecular docking analysis was also performed to determine possible binding modes between PI3Kγ and the target compounds.

 Please wait while we load your content...
Please wait while we load your content...