Interaction of the 5-fluorouracil analog 5-fluoro-2′-deoxyuridine with ‘N’ and ‘B’ isoforms of human serum albumin: a spectroscopic and calorimetric study
Abstract
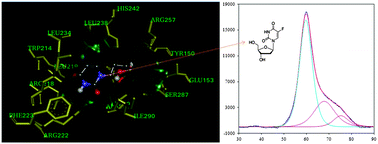
Drugs and metabolites are transported in the blood by plasma proteins, such as human serum albumin (HSA). The uridine analog 2′dFUrd, which is a cytotoxic prodrug metabolite of capecitabine, has remarkable activity against solid tumors when administered orally. We report the results of an in vitro experimental study on the interactions of 2′-dFUrd with the N-isoform (at pH 7.4) and B-isoform (at pH 9.0) of HSA, investigated using fluorescence spectroscopy, circular dichroism (CD), isothermal titration calorimetry (ITC), differential scanning calorimetry (DSC), and molecular docking. The binding constant (Kb) was higher for the N-isoform than for the B-isoform. Thermodynamic parameters, such as enthalpy change (ΔH°), entropy change (ΔS°), and Gibbs free energy change (ΔG°), were also calculated for both isoform interactions using calorimetric techniques. The thermostabilities of HSA and the HSA–2′dFUrd complex were found to be higher for the N-isoform. The interaction of 2′dFUrd with HSA was also explored in molecular docking studies, which revealed that 2′dFUrd was bound to the Sudlow site I in subdomain IIA through multiple modes of interaction, such as hydrophobic interactions and hydrogen bonding. These results suggest that 2′dFUrd has higher binding affinity for the N-isoform of HSA.

 Please wait while we load your content...
Please wait while we load your content...