Renal protein reactivity and stability of antibiotic amphenicols: structure and affinity†
Abstract
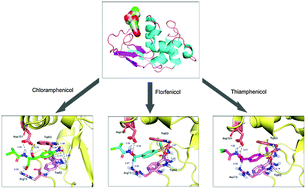
In the present work, the molecular recognition of the oldest active amphenicols by the most popular renal carrier, lysozyme, was deciphered by using fluorescence, circular dichroism (CD) and molecular modeling at the molecular scale. Steady state fluorescence data showed that the recognition of amphenicol by lysozyme yields a static type of fluorescence quenching. This corroborates time-resolved fluorescence results that lysozyme–amphenicol adduct formation has a moderate affinity of 104 M−1, and the driving forces were found to be chiefly hydrogen bonds, hydrophobic interactions and π stacking. Far-UV CD spectra confirmed that the spatial structure of lysozyme was slightly changed with a distinct reduction of α-helices in the presence of amphenicol, suggesting partial destabilization of the protein. Furthermore, via the extrinsic 8-anilino-1-naphthalenesulfonic acid fluorescence spectral properties and molecular modeling, one could see that the amphenicol binding site was situated at the deep crevice on the protein surface, and the ligand was also near to several crucial amino acid residues, such as Trp-62, Trp-63 and Arg-73. Simultaneously, contrastive studies of protein–amphenicols revealed clearly that some substituting groups, e.g. nitryl in the molecular structure of ligands, may be vitally important for the recognition activity of amphenicols with lysozyme. Due to the connection of amphenicols with fatal detrimental effects and because lysozyme has been applied as a drug carrier for proximal tubular targeting, the discussion herein is necessary for rational antibiotic use, development of safe antibiotics and particularly a better appraisal of the risks associated with human exposure to toxic agrochemicals.

 Please wait while we load your content...
Please wait while we load your content...