An efficient Cu(ii)-bis(oxazoline)-based polymer immobilised ionic liquid phase catalyst for asymmetric carbon–carbon bond formation†
Abstract
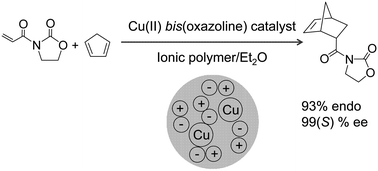
The asymmetric Diels–Alder reaction between N-acryloyloxazolidinone and cyclopentadiene and the Mukaiyama-aldol reaction between methylpyruvate and 1-phenyl-1-trimethylsilyloxyethene have been catalysed by heterogeneous copper(II)-bis(oxazoline)-based polymer immobilised ionic liquid phase (PIILP) systems generated from a range of linear and cross linked ionic polymers. In both reactions selectivity and ee were strongly influenced by the choice of polymer. A comparison of the performance of a range of Cu(II)-bis(oxazoline)-PIILP catalyst systems against analogous supported ionic liquid phase (SILP) heterogeneous catalysts as well as their homogeneous counterparts has been undertaken and their relative merits evaluated.

 Please wait while we load your content...
Please wait while we load your content...