Coupling metal halides with a co-solvent to produce furfural and 5-HMF at high yields directly from lignocellulosic biomass as an integrated biofuels strategy†
Abstract
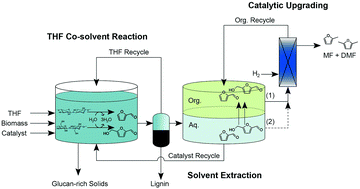
Metal halides are selective catalysts suitable for production of the fuel precursors furfural and 5-HMF from sugars derived from lignocellulosic biomass. However, they do not perform nearly as well when applied to biomass even in combination with immiscible extracting solvents or expensive ionic co-solvents. Here, we couple metal halides with a highly tunable co-solvent system employing renewable tetrahydrofuran (THF) to significantly enhance co-production of furfural and 5-HMF from biomass in a single phase reaction strategy capable of integrating biomass deconstruction with catalytic dehydration of sugars. Screening of several promising metal halide species at 170 °C in pH-controlled reactions with sugar solutions and larger 1 L reactions with maple wood and corn stover revealed how the interplay between relative Brønsted and Lewis acidities was responsible for enhancing catalytic performance in THF co-solvent. Combining FeCl3 with THF co-solvent was particularly effective, achieving one of the highest reported simultaneous yields of furfural (95%) and 5-HMF (51%) directly from biomass with minimal levulinic acid formation (6%). Furthermore, over 90% of the lignin from biomass was extracted by THF and recovered as a fine lignin powder. Tuning the volume ratio of THF to water from 4 : 1 to 1 : 1 preserved 10% to 31% of the reacted biomass as a glucan-rich solid suitable for further catalytic reaction, enzymatic digestion, or possible pulp and paper production.

 Please wait while we load your content...
Please wait while we load your content...