Ag–Fe3O4 nanocomposites@chitin microspheres constructed by in situ one-pot synthesis for rapid hydrogenation catalysis†
Abstract
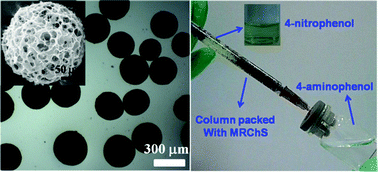
The fabrication of reusable and biodegradable materials from renewable resources such as chitin is essential for a sustainable world. In the present work, chitin was dissolved in 11 wt% NaOH–4 wt% urea aqueous solution via freezing–thawing, and then chitin microspheres (RChS) were prepared by a sol–gel transition method. Subsequently, novel magnetic Ag–Fe3O4@chitin microspheres (MRChS) were constructed successfully by an in situ one-pot synthesis of Ag–Fe3O4 nanoparticles onto the RChS surface. The magnetic chitin microspheres displayed a spherical shape with a 3D-mesh structure, and had a narrow size distribution (150–400 μm). There were many micro- and nano-pores existing in MRChS, and the Ag–Fe3O4 nanoparticles were immobilized through anchoring with the acetyl amine groups of chitin in these pores. The MRChS microspheres were used as a chromatography column packing material for a “catalytic reaction column”, and exhibited highly effective catalytic activity in the rapid transformation from 4-nitrophenol to 4-aminophenol. Moreover, the microspheres displayed a small hysteresis loop and low coercivity, as well as high turnover frequency (at least 10 times) without any loss of catalytic activity. Thus, MRChS could be quickly removed from the water under a magnetic field, leading to easy recycling and reuse. Therefore, this is an environmentally friendly process, and would be highly beneficial to address industrial requirements.

 Please wait while we load your content...
Please wait while we load your content...