Superparamagnetic γ-Fe2O3 nanoparticles as an easily recoverable catalyst for the chemical recycling of PET†
Abstract
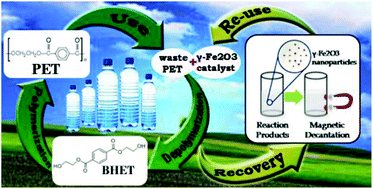
There have been numerous studies to develop catalysts for the chemical recycling of poly(ethylene terephthalate) (PET) via glycolysis. However, in the field of PET glycolysis, only a few have attempted to recover and reuse the catalysts. This research utilized easily recoverable superparamagnetic γ-Fe2O3 nanoparticles as a reusable catalyst for PET glycolysis. γ-Fe2O3 nanoparticles were produced by calcining Fe3O4 nanoparticles prepared by the co-precipitation method. The produced γ-Fe2O3 nanoparticles had an average size of 10.5 ± 1.4 nm, and a very high surface area reaching 147 m2 g−1. Its superparamagnetic property was also confirmed. Glycolysis reactions were carried out, and the γ-Fe2O3 catalysts were recovered after the reactions by simple magnetic decantation. The use of magnetic iron oxide allowed the easy recovery of the catalyst from the glycolysis products. At 300 °C and a 0.05 catalyst/PET weight ratio, the maximum bis(2-hydroxyethlyl) terephthalate (BHET) monomer yield reached more than 90% in 60 min. At 255 °C and a 0.10 catalyst/PET weight ratio, the BHET yield reached more than 80% in 80 min. The catalyst was reused 10 times, giving almost the same BHET yield each time.

 Please wait while we load your content...
Please wait while we load your content...