Facile synthesis of 2-methylenecyclobutanones via Ca(OH)2-catalyzed direct condensation of cyclobutanone with aldehydes and (PhSe)2-catalyzed Baeyer–Villiger oxidation to 4-methylenebutanolides†
Abstract
2-Methylenecyclobutanones (2-MCBones), used to be difficult to access, but can now be easily achieved by a green and stereospecific Ca(OH)2-catalyzed direct and simple aldol condensation of cyclobutanone and aldehydes under mild conditions. The obtained (E)-2-MCBones should be a class of potentially useful building blocks in synthesis as they could readily undergo an interesting (PhSe)2-catalyzed Baeyer–Villiger (BV) oxidation with H2O2 at room temperature to give the versatile 4-methylenebutanolides. Mechanistic studies revealed that the BV reaction most possibly proceeded via the initial formation of benzeneseleninoperoxoic anhydride [PhSe(O)O]2O, which then converted to benzeneseleninoperoxoic acid PhSe(O)OOH as the active oxidant, followed by its selective addition to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of 2-MCBones and then a selective C–C bond cleavage and rearrangement to give 4-methylenebutanolides.
O bond of 2-MCBones and then a selective C–C bond cleavage and rearrangement to give 4-methylenebutanolides.
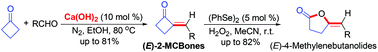

 Please wait while we load your content...
Please wait while we load your content...