An experimental and modeling/simulation-based evaluation of the efficiency and operational performance characteristics of an integrated, membrane-free, neutral pH solar-driven water-splitting system†
Abstract
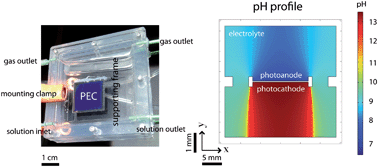
The efficiency limits, gas-crossover behavior, formation of local pH gradients near the electrode surfaces, and safety characteristics have been evaluated experimentally as well as by use of multi-physics modeling and simulation methods for an integrated solar-driven water-splitting system that operates with bulk electrolyte solutions buffered at near-neutral pH. The integrated membrane-free system utilized a triple-junction amorphous hydrogenated Si (a-Si:H) cell as the light absorber, Pt and cobalt phosphate (Co–Pi) as electrocatalysts for the hydrogen-evolution reaction (HER) and oxygen-evolution reaction (OER), respectively, and a bulk aqueous solution buffered at pH = 9.2 by 1.0 M of boric acid/borate as an electrolyte. Although the solar-to-electrical efficiency of the stand-alone triple-junction a-Si:H photovoltaic cell was 7.7%, the solar-to-hydrogen (STH) conversion efficiency for the integrated membrane-free water-splitting system was limited under steady-state operation to 3.2%, and the formation of pH gradients near the electrode surfaces accounted for the largest voltage loss. The membrane-free system exhibited negligible product-recombination loss while operating at current densities near 3.0 mA cm−2, but exhibited significant crossover of products (up to 40% H2 in the O2 chamber), indicating that the system was not intrinsically safe. A system that contained a membrane to minimize the gas crossover, but which was otherwise identical to the membrane-free system, yielded very low energy-conversion efficiencies at steady state, due to low transference numbers for protons across the membranes resulting in electrodialysis of the solution and the consequent formation of large concentration gradients of both protons and buffer counterions near the electrode surfaces. The modeling and simulation results showed that despite the addition of 1.0 M of buffering agent to the bulk of the solution, during operation significant pH gradients developed near the surfaces of the electrodes. Hence, although the bulk electrolyte was buffered to near-neutral pH, the electrode surfaces and electrocatalysts experienced local environments under steady-state operation that were either highly acidic or highly alkaline in nature, changing the chemical form of the electrocatalysts and exposing the electrodes to potentially corrosive local pH conditions. In addition to significant pH gradients, the STH conversion efficiency of both types of systems was limited by the mass transport of ionic species to the electrode surfaces. Even at operating current densities of <3 mA cm−2, the voltage drops due to these pH gradients exceeded the combined electrocatalyst overpotentials for the hydrogen- and oxygen-evolution reactions at current densities of 10 mA cm−2. Hence, such near-neutral pH solar-driven water-splitting systems were both fundamentally limited in efficiency and/or co-evolved explosive mixtures of H2(g) and O2(g) in the presence of active catalysts for the recombination of H2(g) and O2(g).

 Please wait while we load your content...
Please wait while we load your content...