Controlling proton movement: electrocatalytic oxidation of hydrogen by a nickel(ii) complex containing proton relays in the second and outer coordination spheres†
Abstract
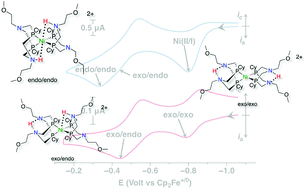
A nickel bis(diphosphine) complex containing proton relays in the second and outer coordination spheres, Ni(PCy2N(CH2)2OMe)2, (PCy2N(CH2)2OMe = 1,5-di(methoxyethyl)-3,7-dicyclohexyl-1,5-diaza-3,7-diphosphacyclooctane), is an electrocatalyst for hydrogen oxidation. The addition of hydrogen to the Ni(II) complex results in rapid formation of three isomers of the doubly protonated Ni(0) complex, [Ni(PCy2N(CH2)2OMe2H)2]2+. The three isomers show fast interconversion at 40 °C, unique to this complex in this class of catalysts. Under conditions of 1.0 atm H2 using H2O as a base, catalytic oxidation proceeds at a turnover frequency of 5 s−1 and an overpotential of 720 mV, as determined from the potential at half of the catalytic current. Compared to the previously reported Ni(PCy2NBn)2 complex, the new complex operates at a faster rate and at a lower overpotential.

 Please wait while we load your content...
Please wait while we load your content...