Axially bonded pentads constructed on the Sn(iv) porphyrin scaffold†
Abstract
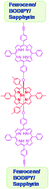
Three covalently linked dyads such as porphyrin–ferrocene, porphyrin–BODIPY and porphyrin–sapphyrin containing one hydroxyphenyl group at the meso-position of porphyrin were synthesized by coupling the trans-functionalized porphyrin building block, 5-(4-hydroxyphenyl)-10-(4-iodophenyl)-15,20-diphenyl porphyrin, with the ethynyl functionalized redox/chromophore building block under mild Pd(0) coupling conditions. The dyads were then reacted with SnTTP(OH)2 in benzene in a 2 : 1 ratio at reflux temperature for 12 hours followed by simple alumina column chromatographic purification which afforded three pentads containing three different types of redox/chromophore components in 62–80% yields. The pentads are very stable and freely soluble in all common organic solvents. The pentads formation were confirmed by MALDI-TOF mass spectrometry and 1D and 2D NMR studies. Absorption and electrochemical studies suggested that the three components in pentads retain their independent characteristic features without significant alterations in their properties thus acting like supramolecular assemblies. The steady state fluorescence studies indicated a possibility of energy/electron transfer among the three types of components in pentads.

 Please wait while we load your content...
Please wait while we load your content...