Synthesis, antiradical activity and in vitro cytotoxicity of novel organotin complexes based on 2,6-di-tert-butyl-4-mercaptophenol†
Abstract
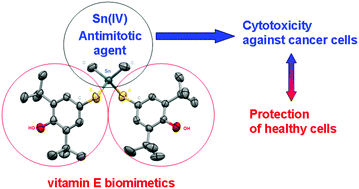
A series of organotin complexes with Sn–S bonds of formulae Me2Sn(SR)2 (1); Et2Sn(SR)2 (2); (n-Bu)2Sn(SR)2 (3); Ph2Sn(SR)2 (4); R2Sn(SR)2 (5); Me3SnSR (6); Ph3SnSR (7) (R = 3,5-di-tert-butyl-4-hydroxyphenyl) were synthesized and characterized by elemental analysis, 1H, 13C NMR, and IR. The crystal structures of compounds 1, 4, 5, and 7 were determined by X-ray diffraction analysis. The tetrahedral geometry around the Sn center in the monocrystals of 1, 4, 5, and 7 was confirmed by X-ray crystallography. The high radical scavenging activity of the complexes was confirmed spectrophotometrically in a DPPH-test. The binding affinity of 1–7 and the starting R2SnCl2 (8) towards tubulin through their interaction with SH groups of proteins was studied. It was found that the hindered organotin complexes could interact with the colchicine site of tubulin, which makes them promising antimitotic drugs. Compounds 1–8 were tested for their in vitro cytotoxicity against human breast (MCF-7) and human cervix (HeLa) adenocarcinoma cells. Complexes 1–8 were also tested against normal human fetal lung fibroblast cells (MRC-5). Complexes 2–4 and 8 exhibit significantly lower cytostatic activity against the normal MRC-5 cell line compared to the tumor cell lines MCF-7 and HeLa used. A high activity against both cell lines 250 nM (MCF-7) and 160 nM (HeLa) was determined for the triphenyltin complex 7 while the introduction of hindered phenol groups decreases the cytotoxicity of the complexes against normal cells.

 Please wait while we load your content...
Please wait while we load your content...