Influence of gold additives on the stability and phase transformation of titanate nanostructures†
Abstract
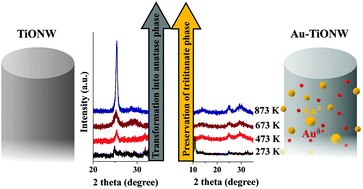
Gold nanoparticles were prepared and characterized on protonated (H-form) titanate nanotubes (TiONTs) and nanowires (TiONWs). The chemical nature and morphology of gold particles were monitored by X-ray photoelectron spectroscopy (XPS), Raman spectroscopy, X-ray diffraction (XRD) and high resolution electron microscopy (HRTEM). The optical properties of Au-containing titanate nanowires were explored by means of ultraviolet-visible diffuse reflectance spectroscopy. The size distribution and homogeneity of gold particles depend on the reduction mode from the corresponding gold salt to metal particles. Smaller clusters (3–8 nm) were obtained with the NaBH4 reactant at 293 K than with molecular hydrogen reduction. An unexpectedly high binding energy gold state was found by XPS in gold-loaded titanate nanostructures. This state was absent from the spectra of gold-loaded TiO2(110). A likely explanation for this phenomenon, supported also by the characteristic decrease of band gap energy from 3.10 eV to 2.74 eV with increasing Au content, is that depending on the metal loading and titanate structure, Au is stabilized on titanate nanowires partially in positively charged gold form by ion exchange and also as Au clusters. Our important new finding is that the thermal annealing behavior of Au-loaded titanate nanotubes and nanowires is different. The former lose their tubular morphology and are readily transformed into anatase even at a very low temperature of 473 K. On the other hand, gold stabilizes the layered structure of titanate nanowires up to 873 K.

 Please wait while we load your content...
Please wait while we load your content...