The effect of the cation alkyl chain length on density and diffusion in dialkylpyrrolidinium bis(mandelato)borate ionic liquids†
Abstract
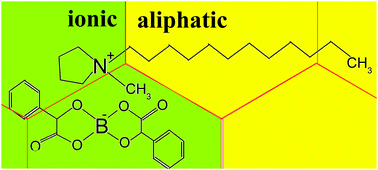
The physicochemical properties of ionic liquids are strongly affected by the selective combination of the cations and anions comprising the ionic liquid. In particular, the length of the alkyl chains of ions has a clear influence on the ionic liquid's performance. In this paper, we study the self-diffusion of ions in a series of halogen-free boron-based ionic liquids (hf-BILs) containing bis(mandelato)borate anions and dialkylpyrrolidinium cations with long alkyl chains CnH2n+1 with n from 4 to 14 within a temperature range of 293–373 K. It was found that the hf-BILs with n = 4–7 have very similar diffusion coefficients, while hf-BILs with n = 10–14 exhibit two liquid sub-phases in almost the entire temperature range studied (293–353 K). Both liquid sub-phases differ in their diffusion coefficients, while values of the slower diffusion coefficients are close to those of hf-BILs with shorter alkyl chains. To explain the particular dependence of diffusion on the alkyl chain length, we examined the densities of the hf-BILs studied here. It was shown that the dependence of the density on the number of CH2 groups in long alkyl chains of cations can be accurately described using a “mosaic type” model, where regions of long alkyl chains of cations (named ‘aliphatic’ regions) and the residual chemical moieties in both cations and anions (named ‘ionic’ regions) give additive contributions. Changes in density due to an increase in temperature and the number of CH2 groups in the long alkyl chains of cations are determined predominantly by changes in the free volume of the ‘ionic’ regions, while ‘aliphatic’ regions are already highly compressed by van der Waals forces, which results in only infinitesimal changes in their free volumes with temperature.

 Please wait while we load your content...
Please wait while we load your content...