The invertible electrochemical properties and thermal response of a series of gel-type ionic liquids based on polyoxometalates†
Abstract
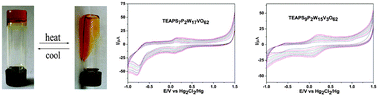
A series of vanadium-substituted Dawson-structure POM-type ionic liquids, [TEAPS]7P2W17VO62 and [TEAPS]9P2W15V3O62, bearing sulfo-group grafted ammonium (TEAPS) cations and Dawson-type polyoxoanions have been formed which are reversible-thermal-response type gels. These gel-type compounds exhibit a phase transition from a quasi-solid gel phase to an isotropic sol phase. What's more, this series of hybrid compounds can undergo reversible electrochemical reactions in dimethyl formamide (DMF) owing to the reduction of the vanadium in POM anions as a simple anion, which is unlikely to happen in water solution because of water protonation.

 Please wait while we load your content...
Please wait while we load your content...