Morphology and surface properties of LiVOPO4: a first principles study†
Abstract
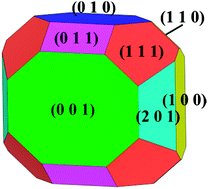
First principles calculations were used to investigate the surface energies, equilibrium morphology, surface redox potentials, and surface electrical conductivity of LiVOPO4. Relatively low-energy surfaces are found in the (100), (010), (001), (011), (111), and (201) orientations of the orthorhombic structure. Thermodynamic equilibrium shape of the LiVOPO4 crystal is built with the calculated surface energies through a Wulff construction. The (001) and (111) orientations are the dominating surfaces in the Wulff shape. Similar calculations for VOPO4 display a larger decrease in surface energies for the (100) surface rather than those in the other surfaces. It suggests that the Wulff shape of LiVOPO4 is closely related to the chemical environment around. Surfaces (100), (010) and (201) present lower Li surface redox potentials in comparison with the bulk material. Therefore, the Li migration rate on surfaces could be effectively increased by maximizing the exposure of these low redox potential surfaces. In addition, lower surface band gaps are found in all orientations compared to the bulk one, which indicates that electrical conductivity can be improved significantly by enlarging surfaces with relatively low band gaps in the particle. Therefore, synthesizing (201) and (100) nanosheets will greatly improve the electrochemical properties of the material.

 Please wait while we load your content...
Please wait while we load your content...