Dual function of a living polymerization initiator through the formation of a chain-end-protecting cluster: density functional theory calculation†
Abstract
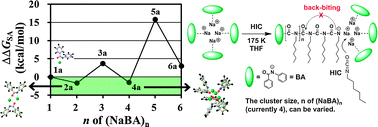
Sodium benzanilide (Na+BA−) initiators have opened a new route to living anionic polymerization of n-hexylisocyanate (HIC) with 100% yield and controlled molecular weight. The NaBA initiators not only provide initiation points for polymerization by attacking HIC monomers but also successfully prevent back-biting side reactions without any help from additives. Our hypothesis on this dual function of the NaBA initiators is that they self-assemble to form protection shields around the chain ends. Indeed, our density functional theory calculations performed under experimental conditions on the free energy of formation of (NaBA)n clusters of various sizes and conformations searched by Monte Carlo simulations show that the BA− moiety forms a stable complex with Na+ in a fan-like circular-sector shape owing to its double binding sites (N−–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ↔ N
O ↔ N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O−) and that the tightly-bound NaBA units spontaneously self-assemble to form small (NaBA)n clusters (n = 2 and 4). The growing end of the polymer chain [(BA)(HIC)n−], which resembles BA−, would also assemble with n − 1 NaBA units to form an n-mer cluster. We expect that the chain end in this cluster would be more available to attack small HIC monomers coming into the cluster (leading to chain growth) rather than folding back to attack the middle of the chain (leading to cyclotrimerization to isocyanurates and depolymerization).
C–O−) and that the tightly-bound NaBA units spontaneously self-assemble to form small (NaBA)n clusters (n = 2 and 4). The growing end of the polymer chain [(BA)(HIC)n−], which resembles BA−, would also assemble with n − 1 NaBA units to form an n-mer cluster. We expect that the chain end in this cluster would be more available to attack small HIC monomers coming into the cluster (leading to chain growth) rather than folding back to attack the middle of the chain (leading to cyclotrimerization to isocyanurates and depolymerization).

 Please wait while we load your content...
Please wait while we load your content...