NO adsorption and reaction on single crystal rutile TiO2(110) surfaces studied using UHV–FTIRS
Abstract
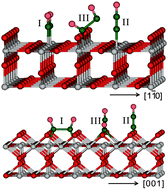
The adsorption and reaction of NO on both the oxidized and reduced single crystal rutile TiO2(110) surfaces were studied in a UHV–FTIRS system at low temperature. The monodentate adsorption configuration of the cis-(NO)2 dimer at bridge oxygen vacancy (Vo) sites was detected for the first time on reduced TiO2(110) surfaces. With the aid of (NO)2 dimer adsorption anisotropy, the bidentate configuration of the cis-(NO)2 dimer on fivefold coordinated Ti5c4+ cation sites was clearly confirmed. The (NO)2 dimer converts to N2O on Ti5c4+ cation sites at higher NO dosage on both oxidized and reduced surfaces, rather than at Vo sites. The (NO)2 → N2O conversion is independent of the presence of Vo on TiO2(110) surfaces. To explain the signs of absorption bands of the dimer monodentate configuration, the local optical constant at Vo sites was introduced.

 Please wait while we load your content...
Please wait while we load your content...